
Practitioner's Corner
J Investig Allergol Clin Immunol 2019; Vol. 29(5): 378-398
© 2019 Esmon Publicidad
derivative skin test was negative. There were no mutations
in the
MEFV, PSTPIP2
, or
IL1RN
genes. Autoantibody titers
(antinuclear antibody, antineutrophil cytoplasmic antibody,
rheumatoid factor) were negative. Echocardiography showed
minimal tricuspid valve regurgitation. Dermatitis herpetiformis
was ruled out by normal small bowel histopathology and
negative antigliadin and antiendomysial antibody titers. The
patient was treated with intravenous antibiotics and discharged
with trimethoprim/sulfamethoxazole prophylaxis.
IgA levels increased and IgG levels decreased over
time (IgG, 598 mg/dL) (Supplementary Material, Table 1).
Findings for lymphocyte proliferation were normal, as were
those for class-switched memory B cells. The patient’s coarse
facial appearance (large ears, broad flat nose, and prominent
forehead) and diffuse xerosis became increasingly evident
(Supplementary Material, Figure 1). At age 4 years, he began
to receive intravenous immunoglobulin therapy to control
recurrent skin and oral lesions following upper respiratory
tract infections. He benefited from regular intravenous
immunoglobulin, although he had severe episodes of oral
mucositis requiring hospitalization twice per year. He also had
peg teeth; his primary teeth had emerged quickly, with rapid
development of caries and short root anomaly (Supplementary
Material, Figure 1).
At age 6 years, a homozygous mutation in the
TTC37
gene (c.2210T>C,p.Val737Ala) was detected by TNGS
with a comprehensive Ion AmpliSeq PID Panel designed for
sequencing 264 PID genes (SupplementaryMaterial, Figure 2).
TTC37
mutations cause THES, which is characterized by
early-onset diarrhea. After the genetic diagnosis, the patient
was reevaluated for THES; liver values were normal, and
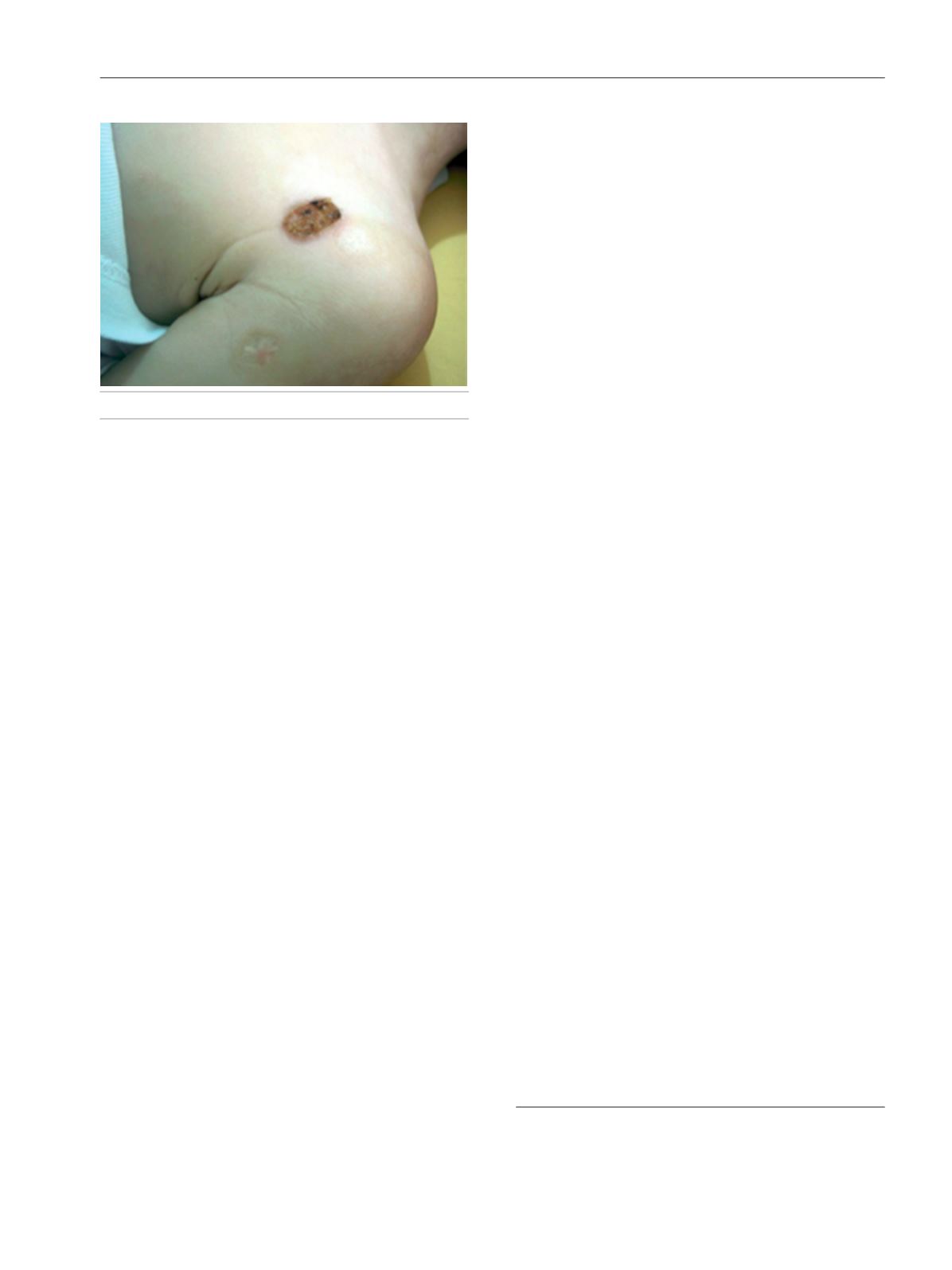
trichorrhexis nodosa was detected in the hair shafts (Figure).
He had mild intermittent diarrhea lasting 2-3 days following
infections. Colonoscopy findings were normal. The parents
were heterozygous for the same mutation.
THES is caused by loss-of-function mutations in the
tetratricopeptide repeat domain–containing protein 37 gene
(
TTC37
) and superkiller viralicidic activity 2 gene (
SKIV2L
)
[3,4]. The condition is characterized by intractable diarrhea,
facial dysmorphism, hair abnormality, intrauterine growth
retardation, immunodeficiency, skin abnormalities, liver
disease, and platelet abnormalities (Supplementary Material,
Table 2) [3-6].
The present case clearly shows that THES can cause
immunodeficiency and pyoderma gangrenosum–like skin
lesions without significant diarrhea. This patient had typical
facial features of THES, wooly and coarse hair, trichorrhexis
nodosa, and hypogammaglobulinemia. He did not have
chronic/intractable diarrhea or liver disease. His height
and weight percentiles were 50%, with normal intelligence
at 7 years of age (Supplementary Material, Figure 3). He also
had peg teeth and short root anomaly. Peg teeth were reported
in a patient with an
SKIV2L
mutation, although they had not
previously been reported in patients with a
TTC37
mutation [6].
Pyoderma gangrenosum is usually associated with systemic
diseases such as inflammatory bowel disease, rheumatologic
disorder, immunodeficiency, or autoinflammation [7,8]. The
presentation we report on here involved recurrent oral aphthous
lesions and pyoderma gangrenosum–like skin eruptions.
Deficiency of IL-1R-antagonist (DIRA) and IL-36R (DITRA)
and PAPA (pyogenic arthritis, pyoderma gangrenosum, acne)
are autoinflammatory disorders with cutaneous pustular
lesions [7,8]. No mutations were found in the
MEFV, PSTPIP2
,
or
IL1RN
genes. Half of all children with THES have cutaneous
abnormalities such as cafe-au-lait spots, xerosis, and rubbery
skin. To our knowledge, there are no previously described cases
of THES presenting with pyoderma gangrenosum.
Approximately90%ofTHEScases have immunodeficiency,
which takes the form of hypogammaglobulinemia, defective
specific antibody production, reduced memory B cell counts,
and abnormal T lymphocyte proliferation [6,9,10]. In the
present case, the patient had selective IgA deficiency at
admission, although he had decreasing IgG levels. IgA levels
returned to normal over time.
The spectrum of THES is widened by pyoderma-like
scarring skin lesions and dental abnormalities, in addition to
classic findings such as immunodeficiency and trichorrhexis
nodosa. To date, mutations have been described in more
than 300 different genes causing primary immunodeficiency
disorders. Diagnosis can be costly and time-consuming
because of the genetic and phenotypic heterogeneity of these
disorders. TNGS enables rapid genetic testing across a large
number of diseases in clinical practice and facilitates the
diagnosis of atypical PID presentations. The power of reverse
phenotyping needs to be emphasized in cases involving
uncertain features or when findings become obvious with age.
Funding
The authors declare that no funding was received for the
present study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
1. Picard C, Bobby Gaspar H,Al-HerzW, Bousfiha A, Casanova JL,
Chatila T, et al. International Union of Immunological Societies:
Figure.
Pyoderma gangrenosum–like skin lesions.
397









