
Omalizumab Response Profile and Management
J Investig Allergol Clin Immunol 2019; Vol. 29(5): 338-348
© 2019 Esmon Publicidad
doi: 10.18176/jiaci.0323
initiation of treatment—our recommendation is to re-evaluate
the patient after 6 months on omalizumab [45]. However, if
the nonresponder shows symptoms of intolerance, therapy
may be changed after 3 months of omalizumab instead of
6 months.
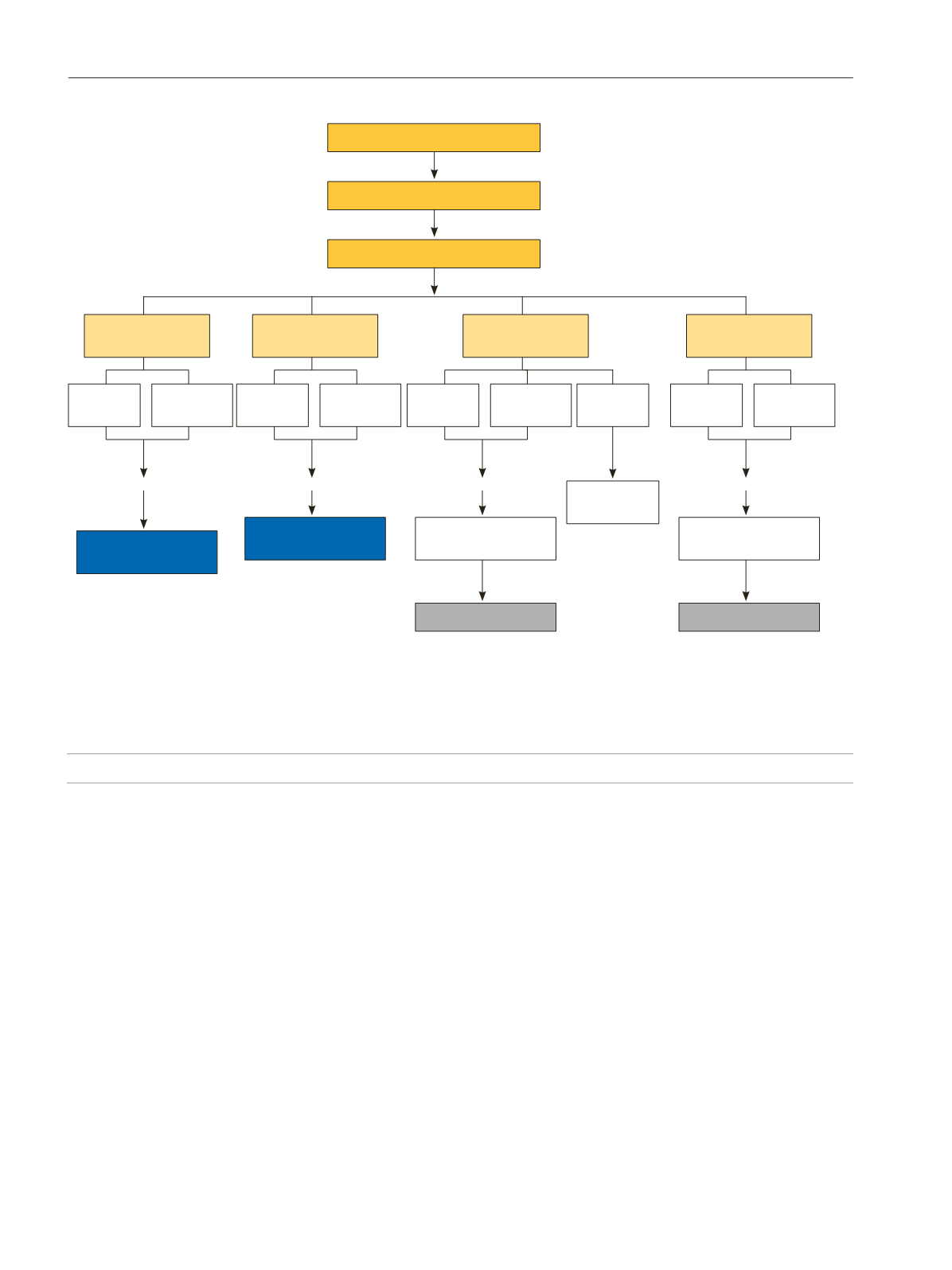
In nonresponders, there are 2 possible therapeutic
strategies: increasing the omalizumab dose while maintaining
the same treatment interval; and reducing the treatment interval
while maintaining the original dose. The strategy selected will
depend on the patient's weekly UAS7 scores over the 4-week
period. Thus, if the UAS7 score remains >16 at all weekly
assessments, then the dose should be increased. However, if
the score is >16 only during 2 weeks after administration, then
the treatment interval should be reduced.
In cases in which the therapeutic strategy is modified, it is
advisable to re-evaluate the patient 3 months after changing the
strategy; if the response does not improve, then we recommend
withdrawing omalizumab and performing another medical
evaluation to reassess the treatment approach.
4.2. Partial Responders
A partial responder to omalizumab is defined a patient
whose UAS7 score partially improves over baseline but who
maintains a UAS7 score of 7-15 (Table 2). In patients who
demonstrate a partial response to the standard omalizumab
dose, we recommend waiting 6 months before altering the
treatment plan, although this will depend on the patient's
symptoms or level of discomfort. If the UAS7 scores remain in
the 7-15–point range after 6 months of standard treatment, we
recommend modifying the regimen. As with nonresponders,
the recommended modification is to either increase the dose
while maintaining the same treatment interval or, conversely,
to shorten the interval from 4 to 2 weeks while maintaining the
original dose. The patient should be re-evaluated after 3months,
and if disease control remains poor, we suggest withdrawing
omalizumab and reassessing the patient. However, it is important
to consider the patient’s opinion with regard to the efficacy of
the drug before deciding to discontinue treatment.
344
Figure 3.
Therapeutic algorithm for the 4 different omalizumab response profiles.
Anti-H
1
(2
nd
generation)
Withdraw omalizumab
and re-evaluation
No response
No response
Worsening?
Worsening?
Review 3 mo
Review 3 mo
Review 3-6 mo** 6 mo
Review 3-6 mo**
3-6 mo
3-6 mo
Review 4 wk
Review 3 and 6 mo*
Review 2-4 wk
↑
2
nd
generation anti-H
1
dose
Omalizumab (300 mg/4 wk)
Withdraw omalizumab
and re-evaluation
Back to dose
300 mg/4wk
Back to dose
300 mg/4wk
Patient re-evaluation
Patient re-evaluation
↑
Dose
= Frequency
↑
Dose
= Frequency
↓
Dose
= Frequency
= Dose
= Frequency
Patient
re-evaluation
↓
Dose
= Frequency
↑
Frequency
= Dose
↑
Frequency
= Dose
↓
Frequency
= Dose
↓
Frequency
= Dose
Nonresponder
UAS7 >16
Partial responder
UAS7= 7-15
Good responder
UAS7= 1-6
Complete responder
UAS7= 0
Short corticosteroid cycles are permitted in exacerbations
*Continue omalizumab up to 6 months, except in nonresponders with intolerable signs and symptoms, and in complete responders, in whom the
therapeutic strategy could be adapted 3 months after initiation of omalizumab.
**In those cases in which a sustained response is achieved for ≥8 weeks, omalizumab can be discontinued to evaluate whether the patient continues
in remission.









