
Giménez Arnau AM, et al.
J Investig Allergol Clin Immunol 2019; Vol. 29(5): 338-348
© 2019 Esmon Publicidad
doi: 10.18176/jiaci.0323
Introduction
Chronic spontaneous urticaria (CSU) is a heterogeneous
condition that causes significant morbidity [1,2]. It is
characterized by the sudden appearance of wheals and/or
angioedema that persist for 6 weeks or longer [2]. In most
cases, the average duration of CSU is from 1 to 5 years [3,4].
CSU is estimated to affect between 0.5% and 1% of the general
population, with an annual frequency of 1.4% [5]. The annual
prevalence of urticaria appears to have increased in recent
years. In Italy, the prevalence increased from 0.02% in 2002 to
0.38% in 2013, with a current incidence rate of 0.10-1.50 per
1000 persons per year [6]. CSU imposes a significant economic
burden and has a substantial negative impact on patient quality
of life (QOL). Therefore, it is crucial to administer effective
treatment as soon as possible [7-9].
The management of CSU consists of a 2-pronged approach
based on avoiding the triggers (if known) and pharmacological
treatment of the symptoms [3]. The current EAACI/GA
2
LEN/
EDF/WAO guidelines recommend second-generation H
1
antihistamines as first-line treatment of the symptoms of
CSU [2,10]. However, given that approximately 70% of
patients remain symptomatic despite the use of antihistamines
at the licensed doses [11,12], the guidelines recommend
increasing the licensed dose by up to 4 times for second-
line treatment [2]. However, a recent systematic review and
meta-analysis estimated that up to 36.8% of patients might
be refractory to the maximum dose of H
1
antihistamines
(4-fold the standard dose) [13]. Recent guidelines recommend
adding omalizumab to treatment with antihistamines as a
third-line treatment. Fourth-line treatment includes the use of
cyclosporineA. For exacerbations, the guidelines recommend
short courses of oral corticosteroids for no more than 10 days
(Figure 1) [2,10].
Phase 3 trials have demonstrated the favorable efficacy
and safety profile of omalizumab [3,14,15], which is
substantially safer than cyclosporine, particularly with regard
to renal toxicity [10]. An expert panel recently drew the same
conclusions regarding the favorable safety and efficacy profile
of omalizumab compared with cyclosporine [16]. In addition,
a recent meta-analysis found that more than 50% of patients
who received cyclosporine at doses of 4-5 mg/kg/d presented
adverse events [17].
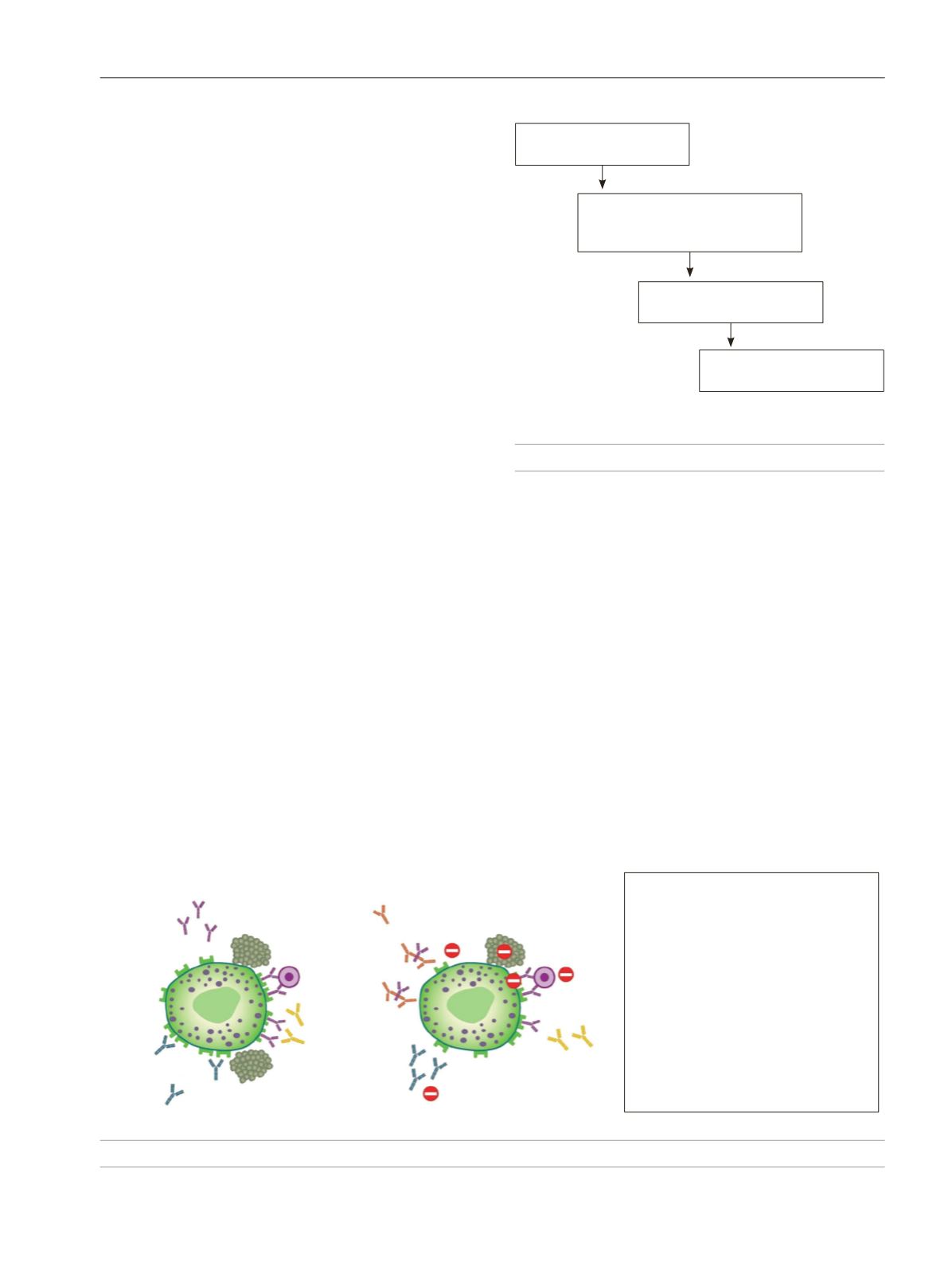
Omalizumab selectively binds to human IgE, thus
preventing binding of IgE to its high-affinity receptor (FcɛRI)
and reducing the amount of free IgE. This process affects
the immunological cascade of urticaria on several levels
(Figure 2) [18,19]. Both the European Medicines Agency and
the United States Food and Drug Administration approved
omalizumab for the treatment of CSU in 2014. The favorable
efficacy and safety data for omalizumab obtained in clinical
trials are further supported by results from real-world clinical
studies [1,20,21]. Available evidence supports the use of
omalizumab for up to 24 weeks as a third-line treatment for
CSU [22]; however, the efficacy of this drug beyond 24 weeks
is less well-established [23]. Although most patients respond
well to omalizumab, the response profile is highly variable
and unpredictable, with some responding quickly and others
responding more slowly or not at all. To date, the different
response profiles have not been well defined, even though clear
339
Figure 1.
Treatment algorithm for chronic spontaneous urticaria.
First line of treatment
Second-generation anti-H
1
Second line of treatment
Dose increase up to 4x the standard
second-generation anti-H
1
dose
Third line of treatment
Omalizumab
Fourth line of treatment
Cyclosporine A
Short corticosteroid cycles may be used (10 days maximum)
to treat exacerbations if necessary
– Reduction in IgE levels
– Dissociation of the IgE-F
cε
RI pre-links
– Reduction in IgE receptors on mast
cells/basophils
– Reduction in mast cell/basophil
degranulation
– Reversion of basopenia and improvement
of the IgE receptor function in basophils
– Reduction in anti-F
cε
RI and anti-IgE
IgG autoantibody activity
– Reduction in antiautoantigen IgE
autoantibodies
Omalizumab
TPO autoantigen
Mast cells/Basophils
IgE
Anti-IgE IgG
Anti-F
cε
RI IgG
F
cε
RI
Figure 2.
Mechanism of action of omalizumab.









