
Kuruvilla M, et al.
J Investig Allergol Clin Immunol 2019; Vol. 29(5): 349-356
© 2019 Esmon Publicidad
doi: 10.18176/jiaci.0320
accompanied by irritability and fatigue [3] and patients with
AC have a poor quality of life, irrespective of the severity
of associated nasal symptoms [10].
Neuronal dysregulation is likely to be responsible for at
least some of these symptoms. Exaggerated hyperreactivity
to nonspecific stimuli such as temperature changes, strong
odors, and irritants is known to be a manifestation of neuronal
inflammation in nonallergic and mixed rhinitis [11]. This is
akin to hyperreactivity to heat, sunlight, and wind during the
active phase of vernal keratoconjunctivitis, which may be
reflective of neural involvement [12], as is the nonspecific
increase in reactivity in the conjunctival response to histamine
in AC patients [13]. In addition, exposure to nonspecific
environmental stimuli, pollutants, and cigarette smoke were
reported to be triggers in a substantial proportion of AC
patients [14] and may be similarly attributable to neural
hypersensitivity. The term vasomotor conjunctivitis has been
used to describe this phenomenon [15].
Mechanisms of AC-Induced Neuropathic
Pain
Sensory Nociceptive Innervation of the Ocular
Surface
Peripheral origin:
The ocular surface is innervated by
primary sensory neurons located in the trigeminal ganglion,
most of which (70%) are polymodal nociceptors [16]. The
afferent C fibers express transient receptor potential (TRP)
channels that play a role in many diseases. Pain and itch
also employ largely overlapping transduction machinery.
Transient receptor potential vanilloid 1 (TRPV1) and transient
receptor potential ankyrin 1 (TRPA1) are 2 such TRP channels
that appear to be important in allergic responses. TRPV1 is
known as a capsaicin responder, but also reacts to a host of
other proinflammatory exogenous and endogenous agents. In
addition, it is stimulated by several mediators that are relevant
to the allergic reaction, such as histamine and bradykinin. As
with TRPV1, TRPA1 is activated by inflammatory mediators
including those involved in allergic disease.
TRPV1/TRPA1 receptor activation in the eye induces the
release of neuropeptides such as neurokinins, calcitonin gene-
related peptide (CGRP), and substance P (SP). Furthermore,
activated sensory neurons can themselves directly release
proinflammatory peptides into surrounding tissue (antidromic
release). Other molecules known as neurotrophins (eg, nerve
growth factor [NGF]), act directly on peptidergic C fiber
nociceptors to potentiate TRPV1 receptors and increase
the expression of substance P and TRPV1. This ultimately
translates into nociception and pain [16].
Central representation:
The cell bodies of sensory neurons
innervating the ocular surface are located in the trigeminal
ganglion and terminate in the trigeminal brainstem complex.
There, they establish contact with second-order ocular neurons
that project to the somatosensory cortex, where the original
noxious signal is perceived as pain.
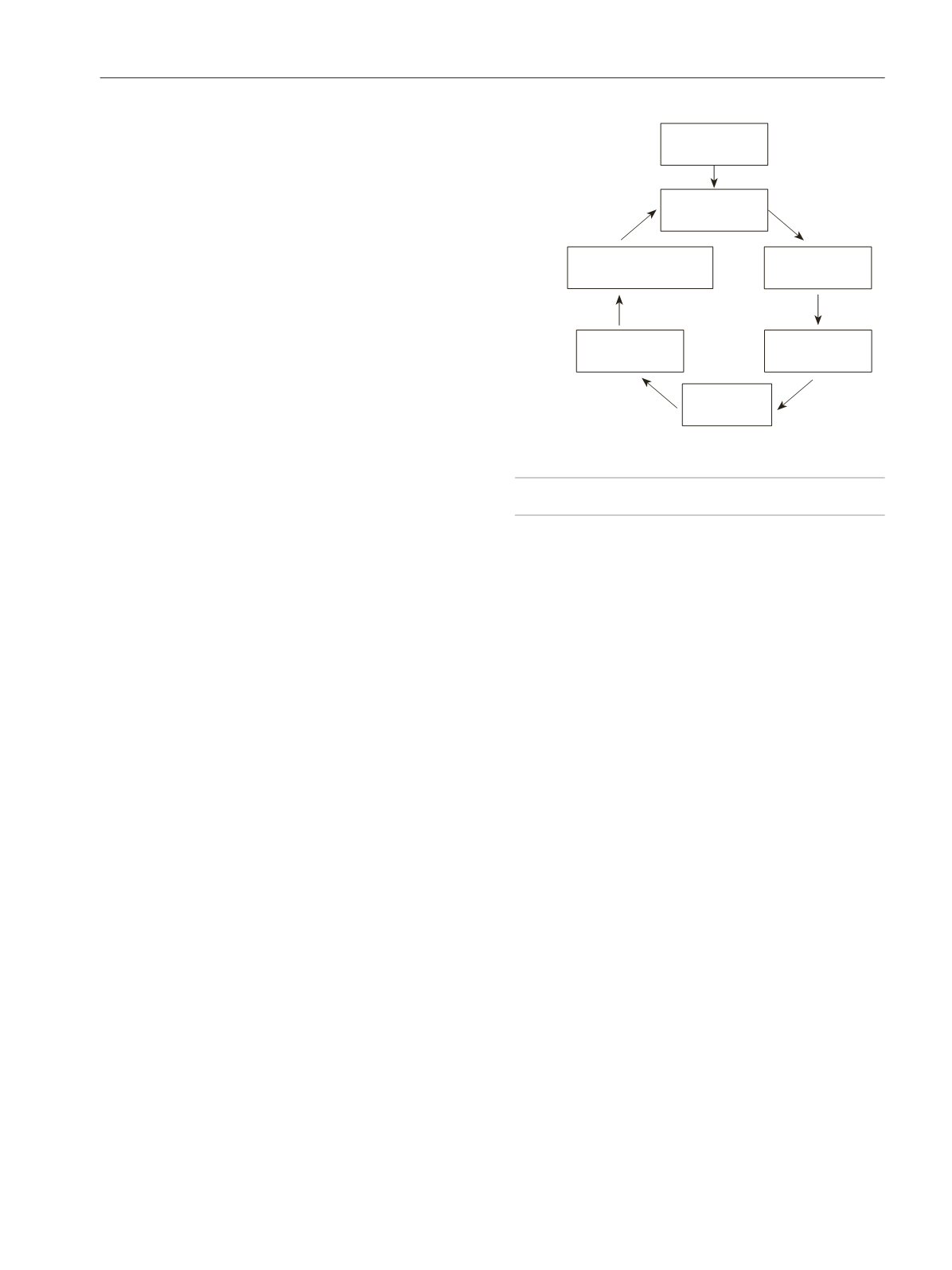
A schematic representation of the pathogenesis of ocular
pain and itch is outlined in Figure 1.
Allergen-Induced Neuromodulation of Sensory Nerves
Under pathological and chronic conditions, dysfunction of
the nervous system itself can generate chronic neuropathic pain
and itch. This is secondary to neural plastic changes in primary
sensory neurons of the peripheral nervous system (peripheral
sensitization) and spinal cord, brainstem, and cortical neurons
in the central nervous system (central sensitization). A
significant body of physiological data suggests that allergy
symptoms may be significantly modulated by the nervous
system. This neural plasticity may be responsible for symptoms
of neuropathic pain and itch in AC. Reflex neural activity
is upregulated in the presence of allergic inflammation and
further amplifies the histamine-mediated immunopathological
response in the conjunctiva.
Peripheral sensitization in allergic inflammation:
During
chronic inflammation, including allergic inflammation, long-
lasting changes develop in the expression and function of
stimulus-transducing ion channels such as TRPV1 and TRPA1.
This results in abnormal hyperexcitability of neurons and may
evoke chronic neuropathic pain.
TRPV1 is believed to be a major cause of neuropathic
pain [17]. It also has a proven role in itch and, in particular,
histamine-induced itch. Chronic allergic inflammation is known
to mediate plasticity of TRPV1 in airway diseases. Inhalation
of allergen by rats or guinea pigs leads to the expression of
TRPV1 in Aδ cough nerves [18]. TRPV1 expression and
substance P levels were found to be significantly higher in
patients with nonallergic rhinitis [19] and asthma, especially
refractory cases [20]. Furthermore, histamine sensitizes the
nociceptor TRPV1 and has been shown to contribute to visceral
hypersensitivity in animals [21]. In addition, other endogenous
inflammatory allergy mediators such as prostaglandin E2 and
bradykinin can markedly enhance the sensitivity of TRPV1
and lower its threshold for activation of sensory nerves [22].
Figure 1.
Schematic representation of neuropathic pain and itch in ocular
surface disease.
Central
sensitization
V1/trigeminal
ganglion
Sensory
cortex
Sensory
plasticity
Increased afferent
excitability
TRPV1
TRPA1
Sub P
NGF
Increased efferent
neural excitability
Noxious
stimulus
Corneal
nociceptors
Peripheral
sensitization
Chronic neuropathic
pain/itch
351









